Research
Background
Sensory Photoreceptors
 Sensory photoreceptor proteins mediate sensation of incident light and enable diverse organisms to derive
spatial and temporal environmental cues for vital adaptations of physiology, behavior and lifestyle.
At the molecular level, sensory photoreceptors comprise photosensor modules that harbor an organic chromophore
and absorb light in the UV/visible region, effector (or, output) modules that exert biological activity, e.g., enzymatic, and
linker elements that connect these modules. Photon absorption by the photosensor initiates a series of mostly reversible photochemical
reactions within the chromophore that couple to the photosensor unit as conformational and dynamic transitions.
These changes propagate through the linker to the effector where they modulate output activity.
Learn less
Sensory photoreceptor proteins mediate sensation of incident light and enable diverse organisms to derive
spatial and temporal environmental cues for vital adaptations of physiology, behavior and lifestyle.
At the molecular level, sensory photoreceptors comprise photosensor modules that harbor an organic chromophore
and absorb light in the UV/visible region, effector (or, output) modules that exert biological activity, e.g., enzymatic, and
linker elements that connect these modules. Photon absorption by the photosensor initiates a series of mostly reversible photochemical
reactions within the chromophore that couple to the photosensor unit as conformational and dynamic transitions.
These changes propagate through the linker to the effector where they modulate output activity.
Learn less
Light-Oxygen-Voltage (LOV) Photoreceptors
Based on chromophore identity and photochemistry, sensory photoreceptors divide into around ten distinct classes. Within the light-oxygen-voltage (LOV) class of blue-light receptors, photon absorption promotes formation of a metastable thioether bond between atom C(4a) of a flavin nucleotide chromophore and atom Sγ of a conserved cysteine within the LOV photosensor module.
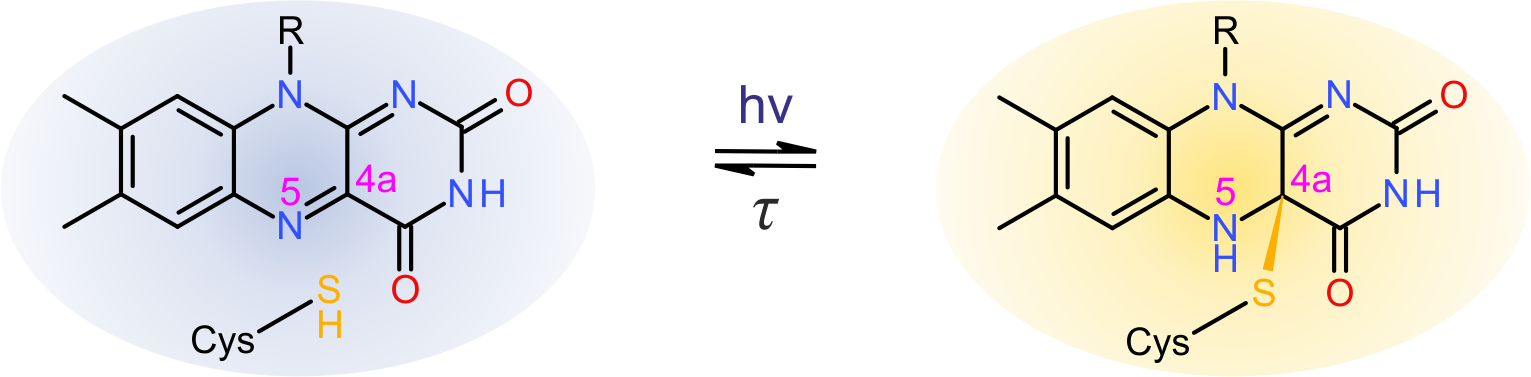 Figure - Simplified photocycle of light-oxygen-voltage photoreceptors.
Figure - Simplified photocycle of light-oxygen-voltage photoreceptors.
Concomitant with bond formation, atom N5 of the flavin is protonated which elicits rearrangements in hydrogen bonding throughout the LOV receptor and enables downstream propagation of the signal. In case of the paradigmatic LOV2 domain of phototropin 1 from Avena sativa these rearrangements culminate in unfolding of an ancillary, C-terminal α helix, denoted Jα.
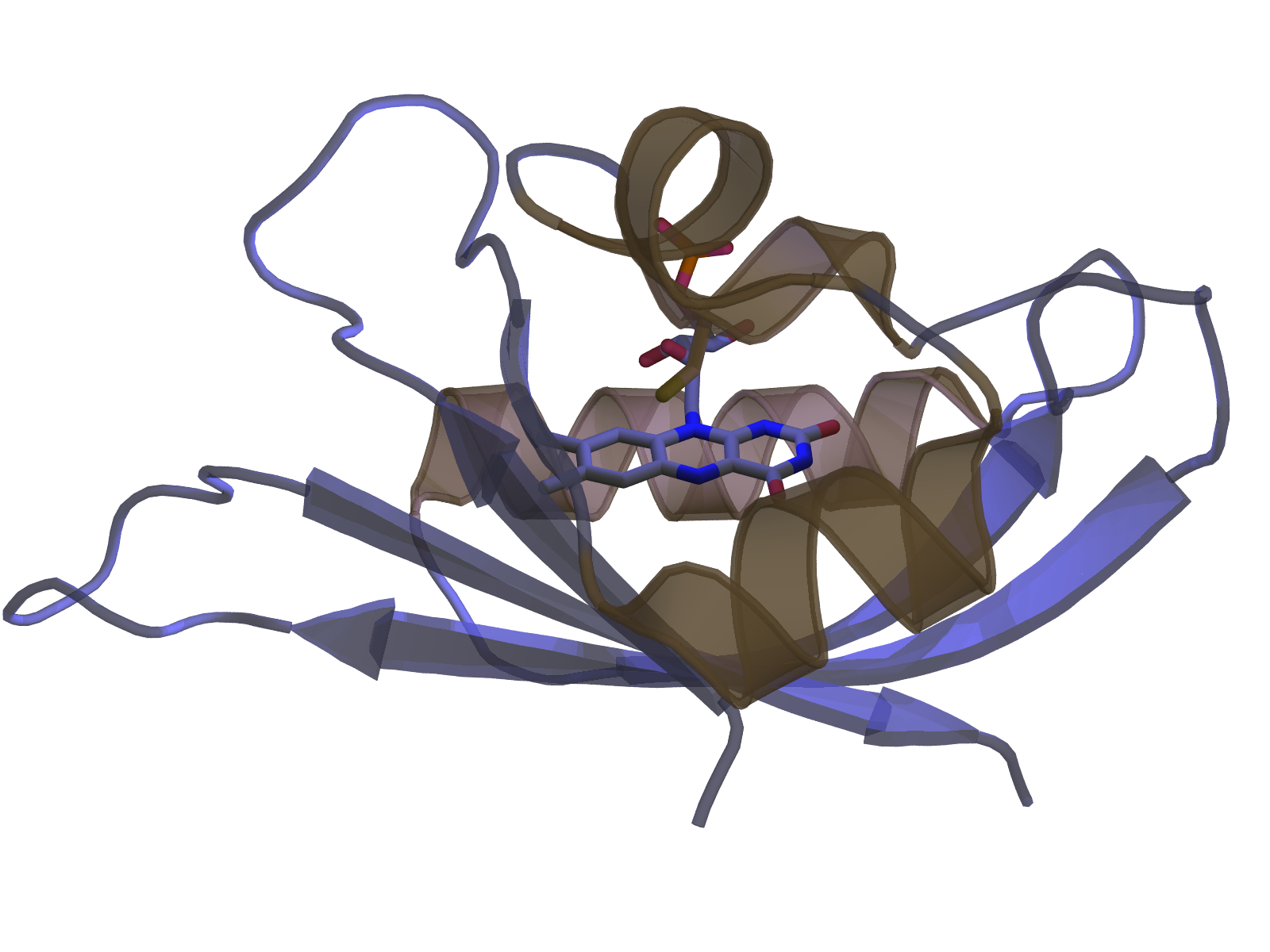
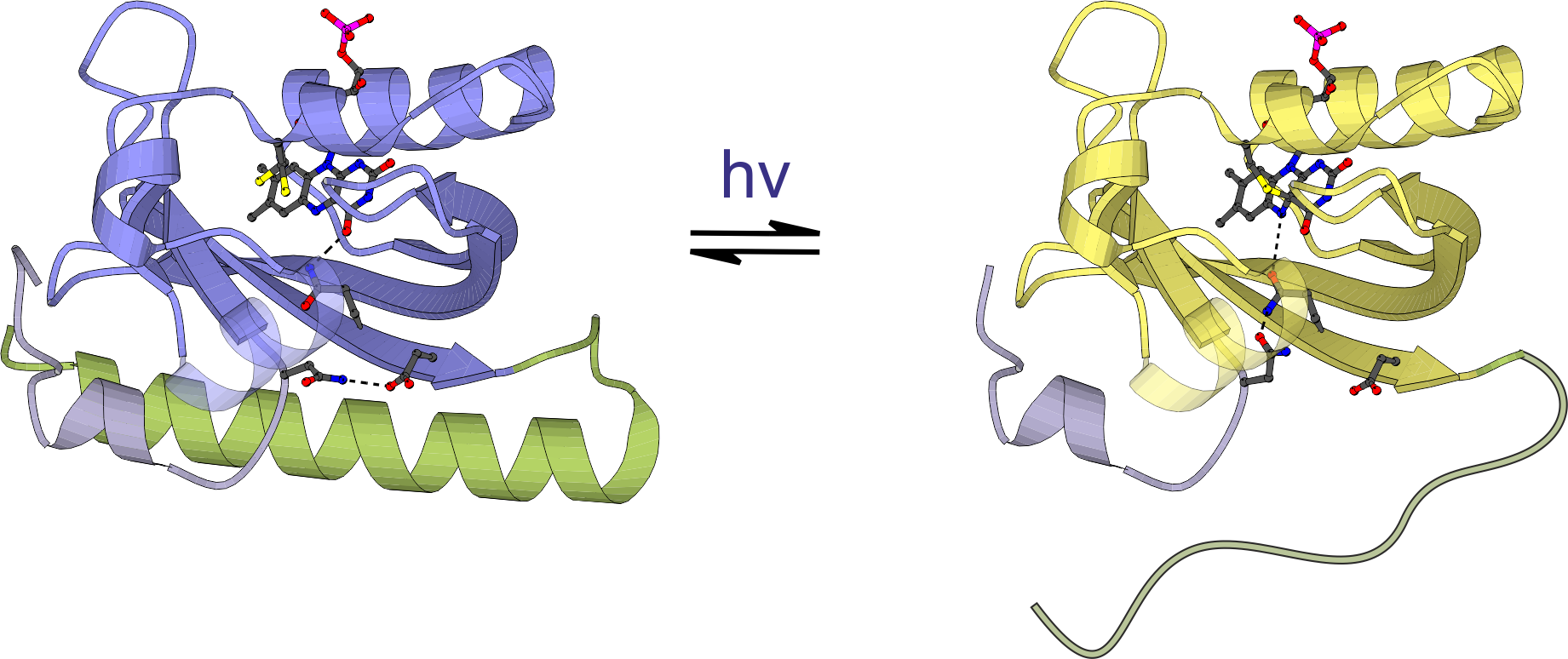 Figure - Blue-light absorption triggers reversible unfolding of the C-terminal Jα helix in the Avena sativa
phototropin 1 LOV2 domain.
Figure - Blue-light absorption triggers reversible unfolding of the C-terminal Jα helix in the Avena sativa
phototropin 1 LOV2 domain.
Sensory photoreceptors usually operate with full reversibility: within the so-called dark-recovery reaction, the receptor returns to the resting state with kinetics that depend on chromophore environment, temperature and solvent composition. Despite essentially the same photochemistry, a palette of LOV photosensors achieve quite distinct structural responses, including order-disorder transitions, association-dissociation reactions and other changes in tertiary and quaternary structure.
- Möglich, A., Yang, X., Ayers, R.A., and Moffat, K. (2010). Structure and function of plant photoreceptors. Annu. Rev. Plant Biol. 61, 21–47. [Pubmed]
- Conrad, K.S., Manahan, C.C., and Crane, B.R. (2014). Photochemistry of flavoprotein light sensors. Nat. Chem. Biol. 10, 801–809. [Pubmed]
Phytochrome Photoreceptors
In contrast to LOV receptors, phytochromes from plants, (cyano)bacteria and fungi utilize linear tetrapyrroles (bilins) as chromophores to sense light in the red/far-red spectral regions. Photon absorption drives a reversible Z/E isomerization around the C15=C16 double bond of the bilin cofactor. Notably, phytochromes are photochromic in that light of one color drives the Z→E transition and light of a different color drives the reverse E→Z transition. Usually, the Z isomer maximally absorbs red light and is hence denoted Pr; by contrast, the E isomer maximally absorbs far-red light and is denoted Pfr. In conventional phytochromes, the Z isomer is the thermodynamically more stable one that persists in the absence of light; in bathyphytochromes it is the E isomer.
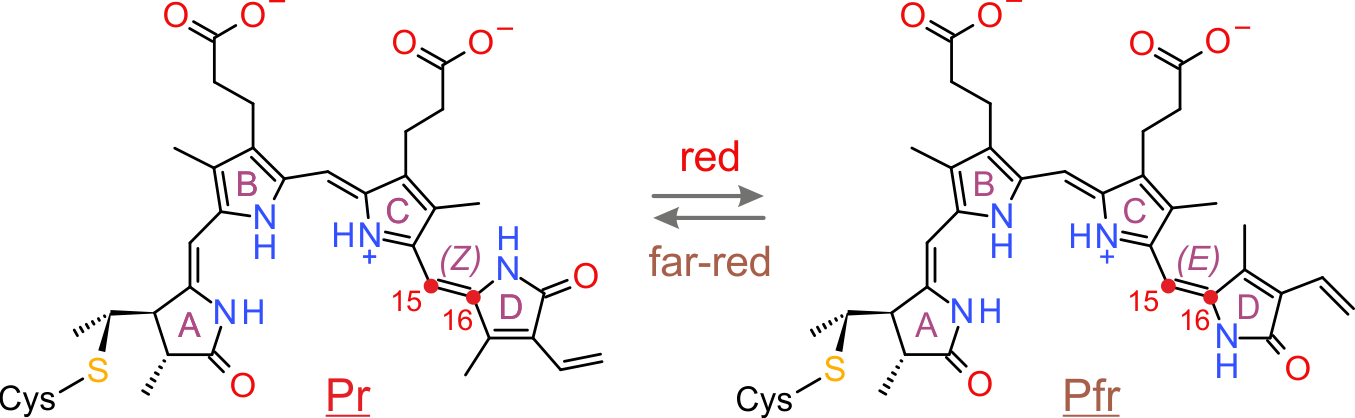 Figure - Simplified photocycle of a plant phytochrome. Red and far-red light drive the reversible Z/E isomerization
around the C15=C16 double bond of a bilin chromophore.
Figure - Simplified photocycle of a plant phytochrome. Red and far-red light drive the reversible Z/E isomerization
around the C15=C16 double bond of a bilin chromophore.
The photosensor module of (most) phytochromes consists of tandem Per-ARNT-Sim (PAS), GAF and PHY domains. The bilin chromophore is covalently bound to a cysteine residue within the PAS domain (bacterial phytochromes) or the GAF domain (plant phytochromes). Light-driven Z/E isomerization promotes refolding of a tongue region emanating from the PHY domain from β-sheet (Pr) to α-helical (Pfr) conformation.
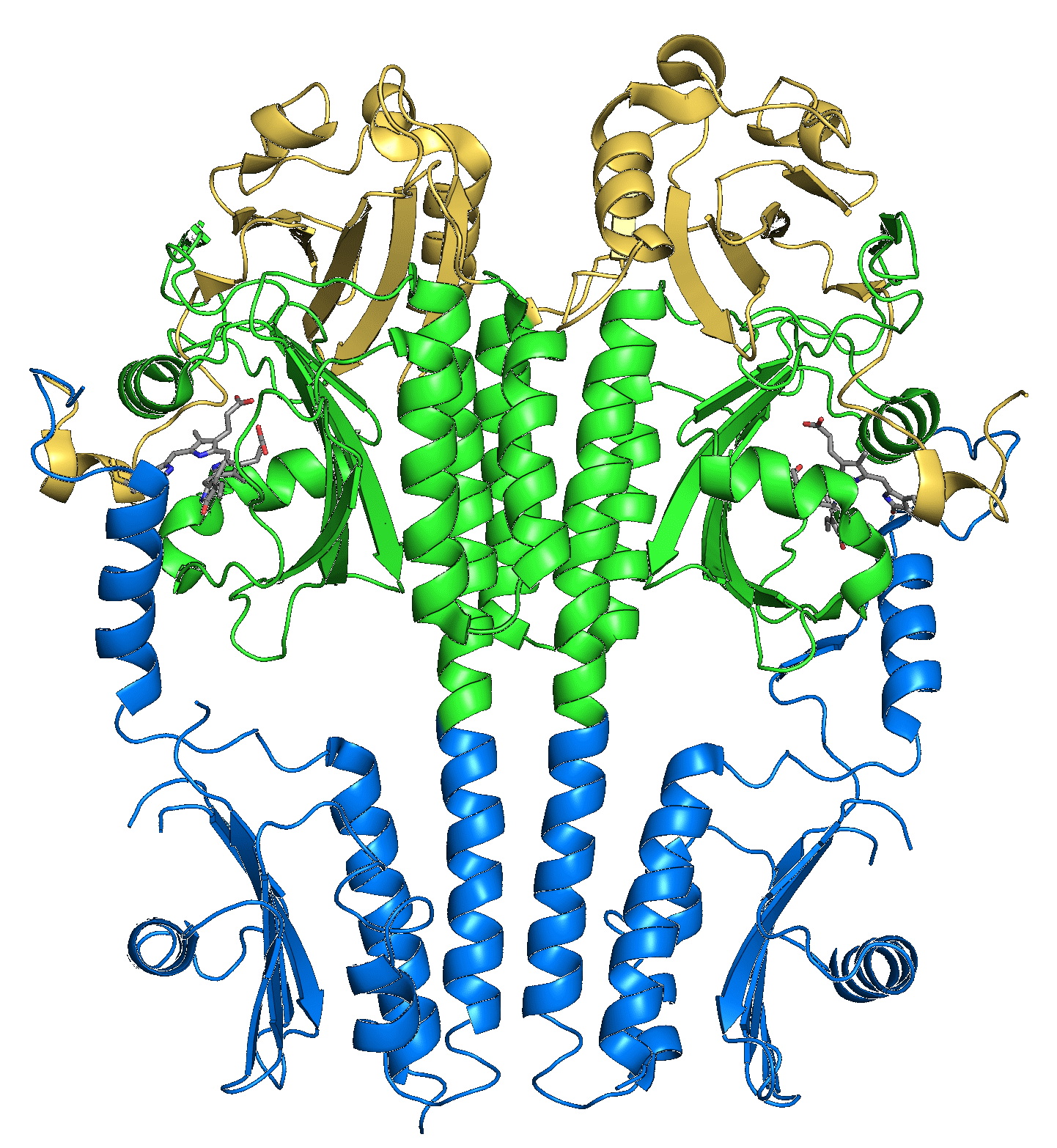 Figure - Structure of the photosensory core (PAS-GAF-PHY) of the Pseudomonas aeruginosa bathy bacteriophytochrome.
Figure - Structure of the photosensory core (PAS-GAF-PHY) of the Pseudomonas aeruginosa bathy bacteriophytochrome.
- Rockwell, N.C., and Lagarias, J.C. (2010). A brief history of phytochromes. Chemphyschem 11, 1172–1180. [Pubmed]
Optogenetics & Photoreceptor Engineering
 Sensory photoreceptors excel in the spatial and temporal precision of the responses they mediate. For exactly these reasons, photoreceptors have also
found use in optogenetics as light-gated protein actuators. Optogenetics denotes the deployment of genetically encoded agents to monitor or control by light
cellular events. Initially confined to the neurosciences, optogenetics has since greatly transcended its initial domain and now enables light-mediated intervention
in numerous cellular processes. In particular, the engineering of novel photoreceptors with customized response to light has unlocked additional areas for optogenetics.
Learn less
Sensory photoreceptors excel in the spatial and temporal precision of the responses they mediate. For exactly these reasons, photoreceptors have also
found use in optogenetics as light-gated protein actuators. Optogenetics denotes the deployment of genetically encoded agents to monitor or control by light
cellular events. Initially confined to the neurosciences, optogenetics has since greatly transcended its initial domain and now enables light-mediated intervention
in numerous cellular processes. In particular, the engineering of novel photoreceptors with customized response to light has unlocked additional areas for optogenetics.
Learn less
Photoreceptor Engineering
Conceptually simple, the engineering of sensory photoreceptors amounts to the recombination of photosensor modules with the desired light sensitivity and effector modules with the desired biological output function. However, the challenge lies in precisely linking these modules such that they are thermodynamically coupled, that is, that light absorption within the photosensor can indeed be transmitted to the effector and be converted into appropriate changes of biological activity.
Photosensor
Effector
Empowered by a detailed understanding of the molecular properties of natural photoreceptors, the engineering of photoreceptors has been remarkably successful and to date numerous light-gated actuators with customized traits have become available that can be deployed in optogenetics. Despite the diversity of engineered photoreceptors, successful design strategies can be grouped in three clades. First, certain photoreceptors naturally undergo light-dependent oligomerization reactions which are harnessed for the construction of light-gated protein-protein interactions. Second, light-driven unfolding reactions in other photoreceptors are suitable for displaying or sequestering peptide epitopes as a function of light. Third, a diverse group comprises several modes of light-driven changes in tertiary and quaternary structure (other than unfolding and association reactions).
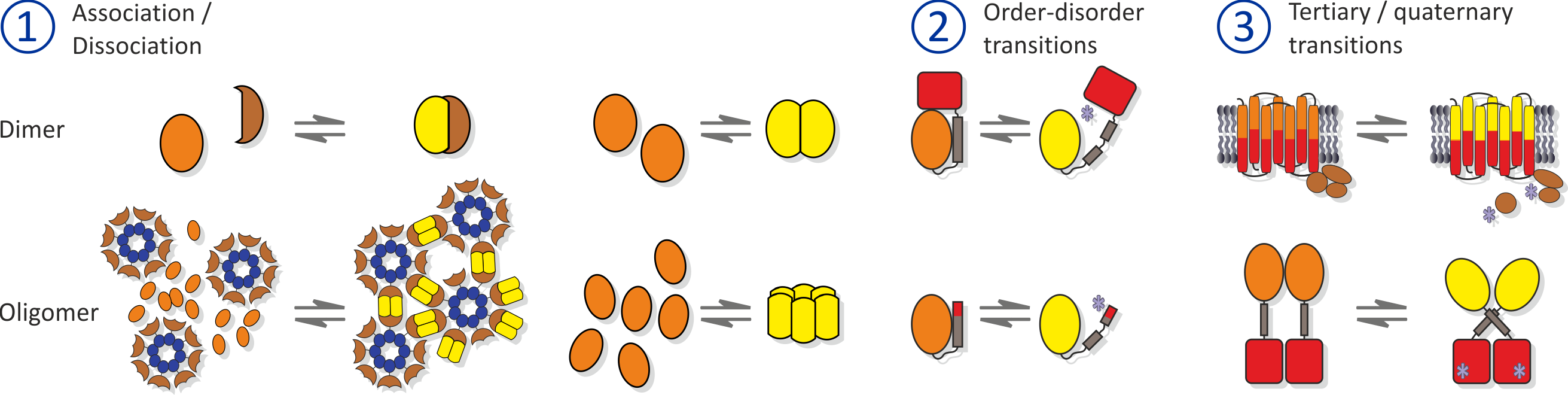 Figure - Approaches for photoreceptor engineering.
1. Light-regulated association/dissociation reactions. 2. Light-regulated unfolding reactions. 3. Other forms of light-induced allostery.
Figure - Approaches for photoreceptor engineering.
1. Light-regulated association/dissociation reactions. 2. Light-regulated unfolding reactions. 3. Other forms of light-induced allostery.
- Möglich, A., and Moffat, K. (2010). Engineered photoreceptors as novel optogenetic tools. Photochem. Photobiol. Sci. 9, 1286–1300. [Pubmed]
- Ziegler, T., and Möglich, A. (2015). Photoreceptor engineering. Front. Mol. Biosci. 2, 30. [Pubmed]
- Nack, J., and Möglich, A. (2017). Optische Kontrolle zellulärer Prozesse. Nachr. Chem. 65, 309-313. [doi]
Research Projects
Light-regulated Histidine Kinases - Engineering
 Sensor histidine kinases (SHK) form part of two-component systems (TCS) which represent the predominant means by which diverse prokaryotes sense and interact with their
environment. In classic TCS, the SHK autophosphorylates at a conserved histidine residue and then transfers the phosphoryl group to a so-called response regulator (RR);
both phosphorylation events are regulated by the cognate signal of the SHK, e.g., the concentration of certain metabolites or physical stimuli like temperature or light.
In its phosphorylated state, the RR elicits downstream responses, most often activation of gene expression from specific promotors. Exploiting the modular architecture
of signal receptors, we have engineered SHKs that respond to blue light.
Learn less
Sensor histidine kinases (SHK) form part of two-component systems (TCS) which represent the predominant means by which diverse prokaryotes sense and interact with their
environment. In classic TCS, the SHK autophosphorylates at a conserved histidine residue and then transfers the phosphoryl group to a so-called response regulator (RR);
both phosphorylation events are regulated by the cognate signal of the SHK, e.g., the concentration of certain metabolites or physical stimuli like temperature or light.
In its phosphorylated state, the RR elicits downstream responses, most often activation of gene expression from specific promotors. Exploiting the modular architecture
of signal receptors, we have engineered SHKs that respond to blue light.
Learn less
Engineering of YF1
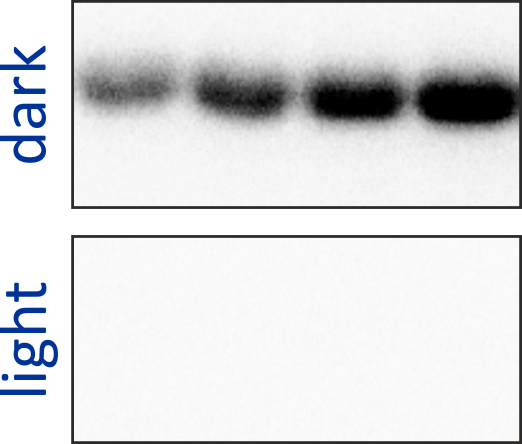
Originally motivated by the structural homology between the sensor domains of the blue-light receptor YtvA from Bacillus subtilis (BsYtvA) and the oxygen-sensitive sensor histidine kinase FixL from Bradyrhizobium japonicum (BjFixL), we reasoned that these modules are interchangeable. Replacement of the PAS-A and the heme-binding, oxygen-sensing PAS-B domain of BjFixL with the LOV photosensor domain of BsYtvA subjected catalytic activity of the resultant histidine kinase YF1 under blue-light control. Phosphorylation of the response regulator BjFixJ was repressed by more than 1000-fold in blue light compared to darkness.
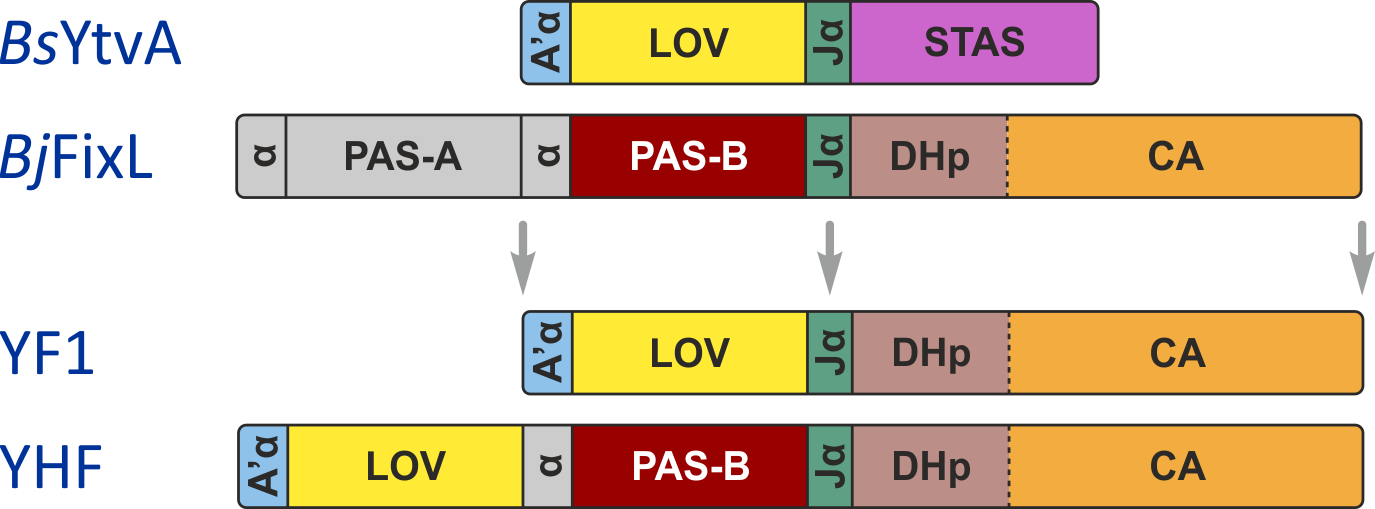
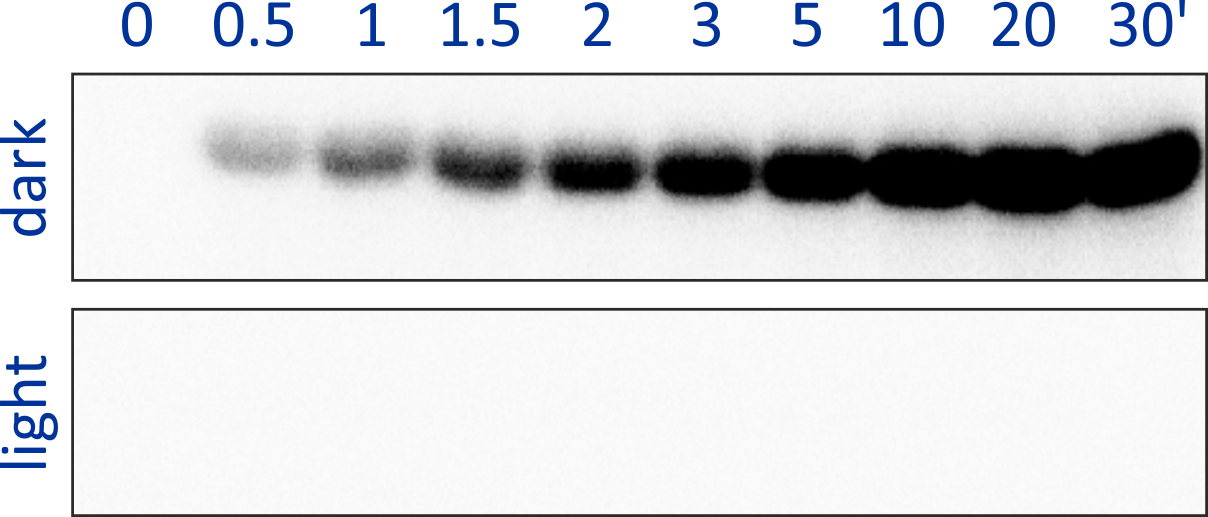 Figure - The engineered histidine kinases YF1 and YHF have been constructed via modular recombination
of sensor and effector modules of the parental receptors BsYtvA and BjFixL. Over the time course of 30 minutes, YF1 readily phosphorylates its response
regulator BjFixJ in light-dependent manner.
Figure - The engineered histidine kinases YF1 and YHF have been constructed via modular recombination
of sensor and effector modules of the parental receptors BsYtvA and BjFixL. Over the time course of 30 minutes, YF1 readily phosphorylates its response
regulator BjFixJ in light-dependent manner.
- Möglich, A., and Moffat, K. (2007). Structural Basis for Light-dependent Signaling in the Dimeric LOV Domain of the Photosensor YtvA. J. Mol. Biol. 373, 112–126. [Pubmed]
- Möglich, A., Ayers, R.A., and Moffat, K. (2009). Design and signaling mechanism of light-regulated histidine kinases. J. Mol. Biol. 385, 1433–1444. [Pubmed]
Signal Integration in Sensor Histidine Kinases
Replacement of PAS-A of BjFixL with BsYtvA LOV, that is, retaining PAS-B, yielded the histidine kinase YHF whose activity is regulated by the two signals blue light and oxygen in positive cooperative manner. Whereas blue light and oxygen separately only attenuated kinase activity by around 2- to 3-fold, the combination of both signals resulted in a much more pronounced reduction of catalytic activity.
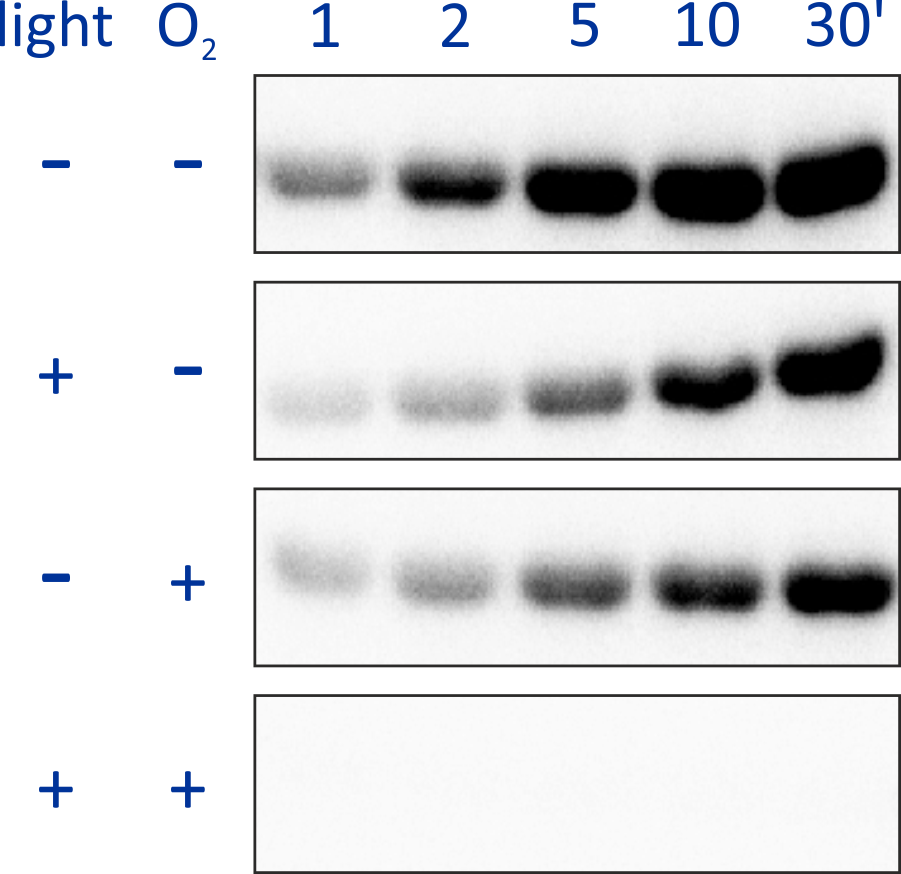 Figure - The engineered histidine kinase YHF comprises both LOV blue-light and PAS-B oxygen sensor
domains. The signals blue light and oxygen are sensed and integrated in positive cooperative manner: the combined effect of both signals much exceeds those for
either signal separately.
Figure - The engineered histidine kinase YHF comprises both LOV blue-light and PAS-B oxygen sensor
domains. The signals blue light and oxygen are sensed and integrated in positive cooperative manner: the combined effect of both signals much exceeds those for
either signal separately.
- Möglich, A., Ayers, R.A., and Moffat, K. (2010). Addition at the molecular level: signal integration in designed Per-ARNT-Sim receptor proteins. J. Mol. Biol. 400, 477–486. [Pubmed]
Architecture of Signal Receptors
Certain of the underlying design principles that underlie the construction of YF1 and YHF are also realized in numerous natural signal receptors. As exemplified for PAS-GGDEF proteins, diverse receptor families feature linker elements with conserved patterns of hydrophobicity and discrete lengths, indicative of α-helical conformation.
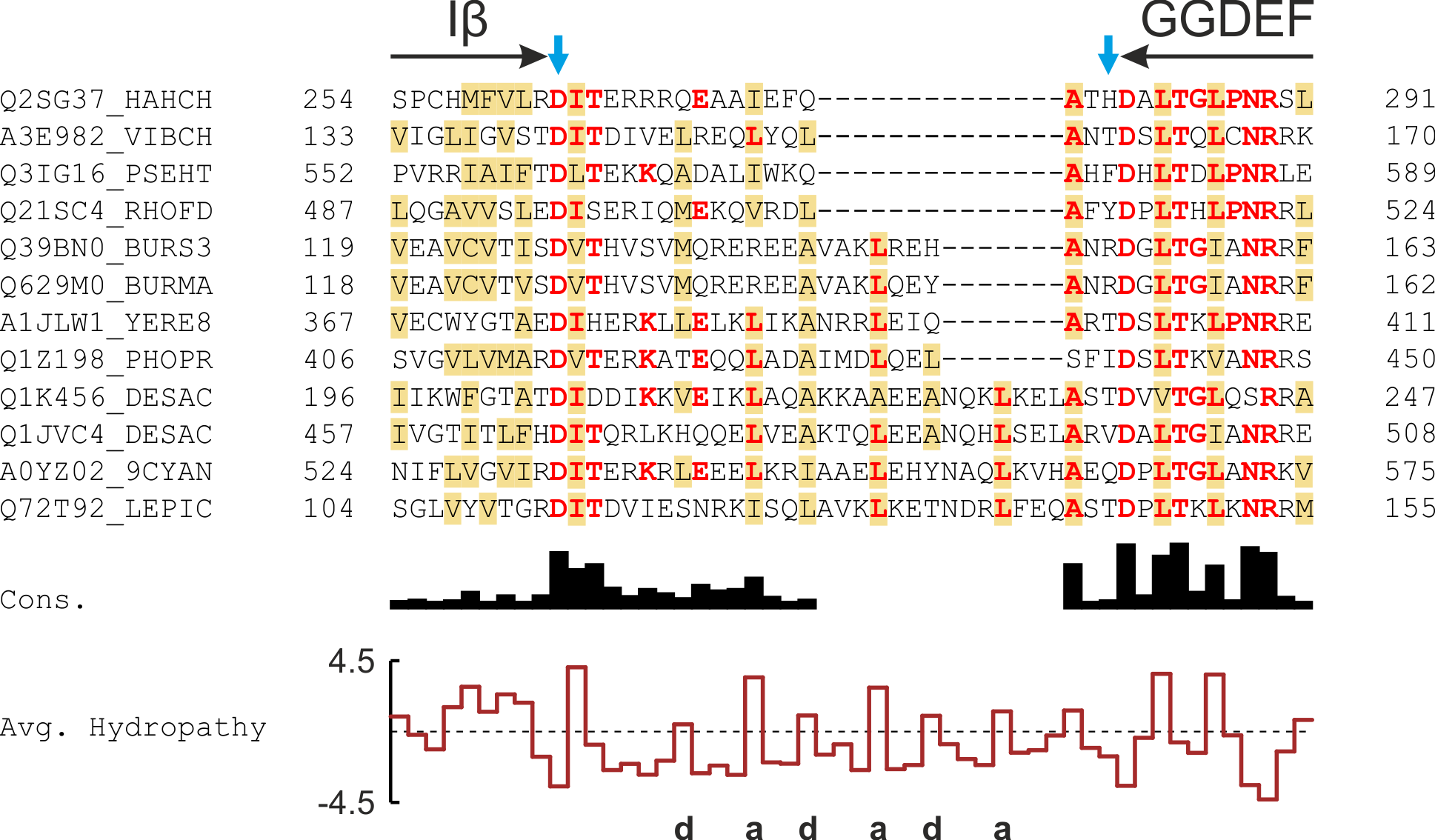
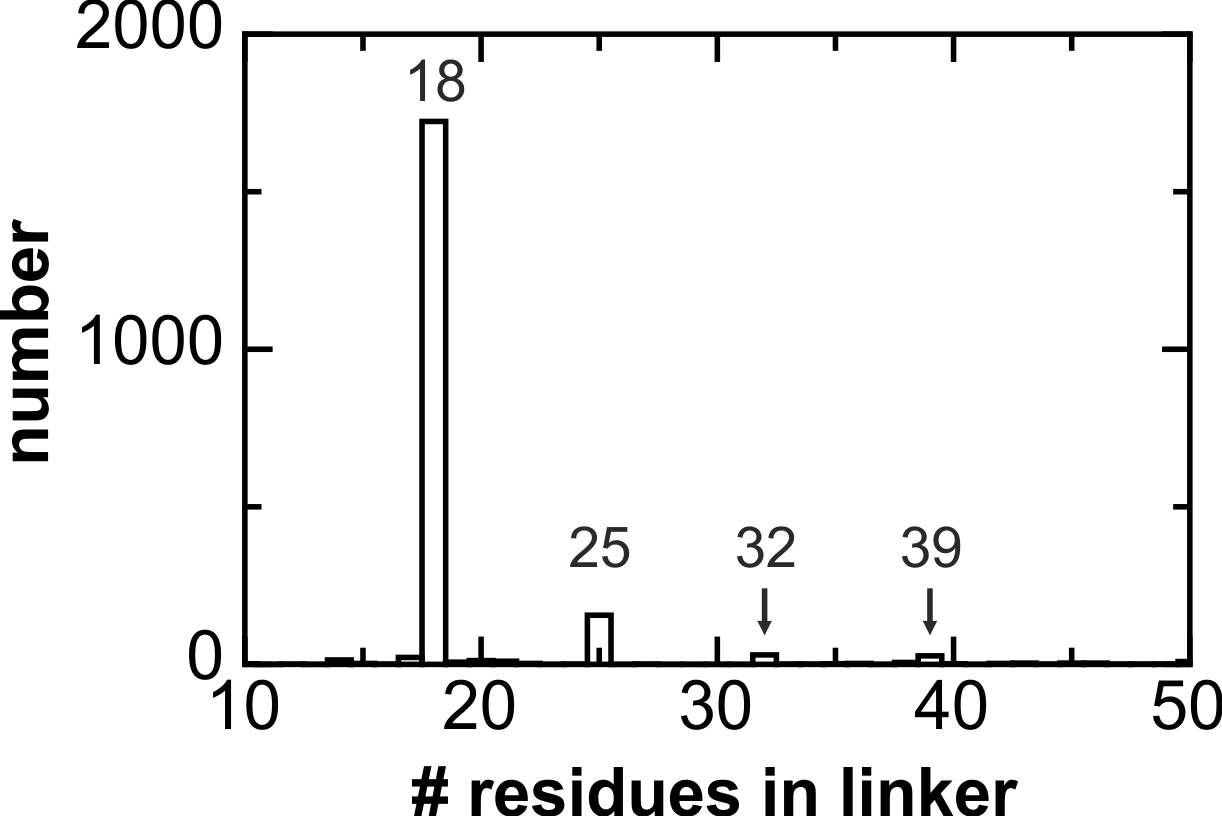 Figure - The multiple sequence alignment of the linkers between PAS and GGDEF domains reveals periodic
patterns of hydrophobicity and highly discretized length distributions.
Figure - The multiple sequence alignment of the linkers between PAS and GGDEF domains reveals periodic
patterns of hydrophobicity and highly discretized length distributions.
- Möglich, A., Ayers, R.A., and Moffat, K. (2009). Design and signaling mechanism of light-regulated histidine kinases. J. Mol. Biol. 385, 1433–1444. [Pubmed]
- Möglich, A., Ayers, R.A., and Moffat, K. (2009). Structure and signaling mechanism of Per-ARNT-Sim domains. Structure 17, 1282–1294. [Pubmed]
- Ohlendorf, R., Schumacher, C.H., Richter, F., and Möglich, A. (2016). Library-Aided Probing of Linker Determinants in Hybrid Photoreceptors. ACS Synth. Biol. 5, 1117-1126. [Pubmed]
- Stabel, R., Stüven, B., Ohlendorf, R., and Möglich, A. (2017). Primer-Aided Truncation for the Creation of Hybrid Proteins. Meth. Mol. Biol. 1596, 287-304. [Pubmed]
Light-regulated Histidine Kinases - Structure & Mechanism
 To obtain a molecular view of signal transduction, we have determined the crystal structure of the engineered, blue-light-regulated histidine kinase YF1 in its
dark-adapted state. In the homodimeric receptor, two LOV photosensor modules form a parallel dimer that is connected via an α-helical coiled coil to the
effector modules. The high-resolution structure of YF1 provides a backdrop for devising and rationalizing photoreceptor variants. The structure of the transiently
populated light-adapted state of YF1 is interrogated by electron paramagnetic resonance spectroscopy and solution X-ray scattering, respectively.
Learn less
To obtain a molecular view of signal transduction, we have determined the crystal structure of the engineered, blue-light-regulated histidine kinase YF1 in its
dark-adapted state. In the homodimeric receptor, two LOV photosensor modules form a parallel dimer that is connected via an α-helical coiled coil to the
effector modules. The high-resolution structure of YF1 provides a backdrop for devising and rationalizing photoreceptor variants. The structure of the transiently
populated light-adapted state of YF1 is interrogated by electron paramagnetic resonance spectroscopy and solution X-ray scattering, respectively.
Learn less
Dark-adapted Structure of YF1
The dark-adapted structure of YF1 was resolved in its entirety by X-ray crystallography. In the homodimeric photoreceptor, two LOV photosensor domains embrace a short α-helical coiled coiled formed by the N-terminal A'α extensions. The C-terminal Jα extensions form a coaxial, parallel coiled coil that links to the histidine kinase effector which comprises DHp and CA domains. Evidently, photosensor and effector are spatially separated, thus not in direct contact and apparently they communicate through the coiled-coil linker.
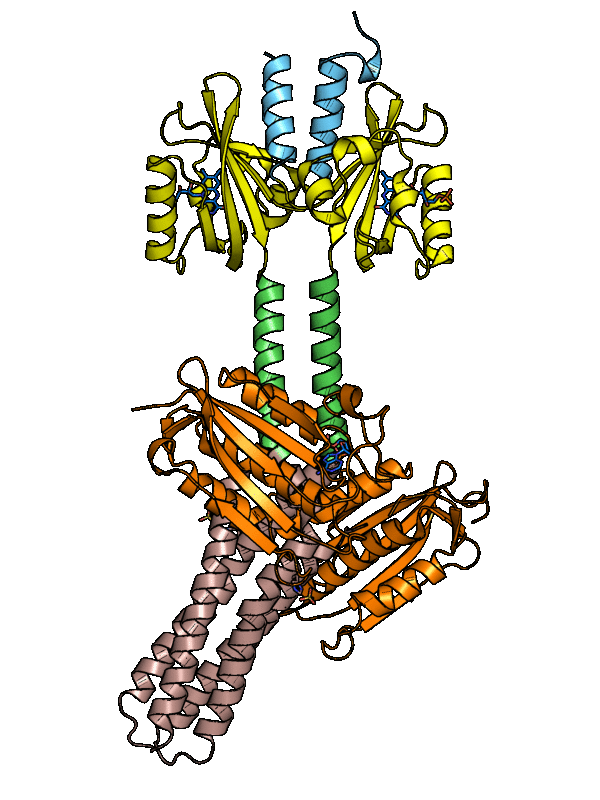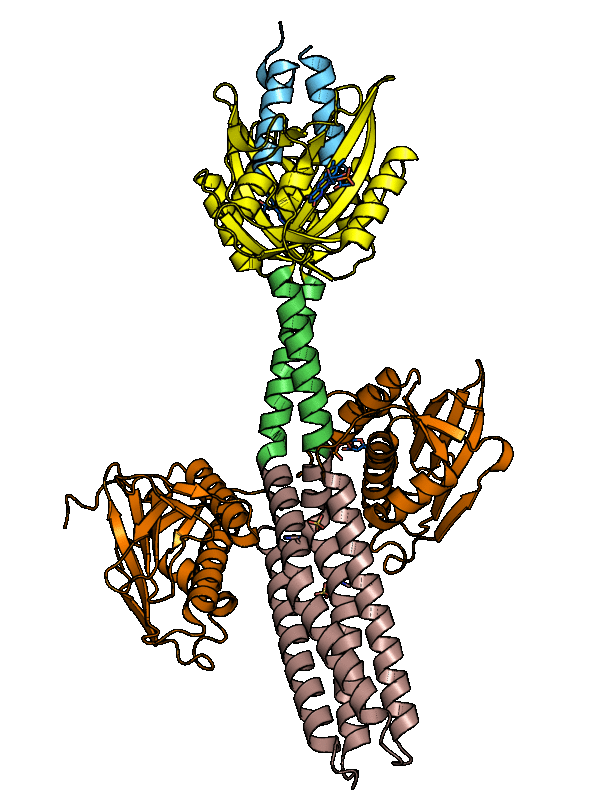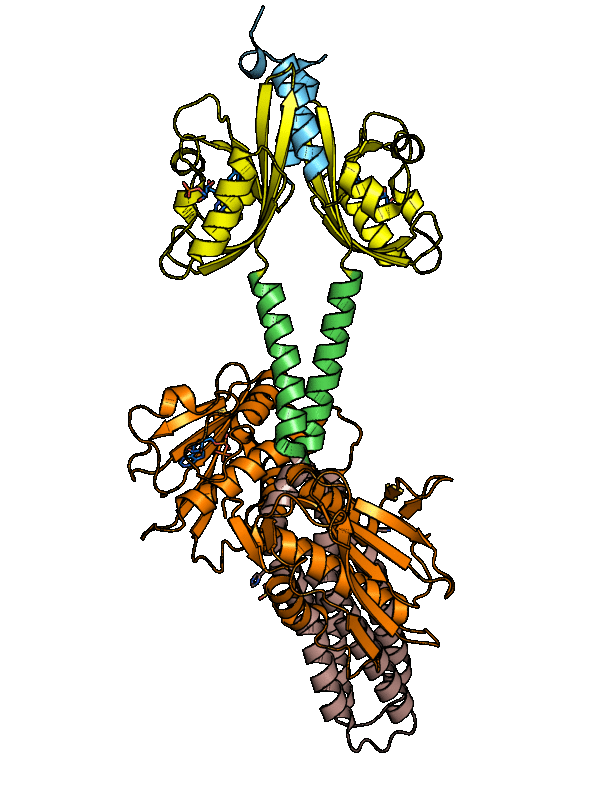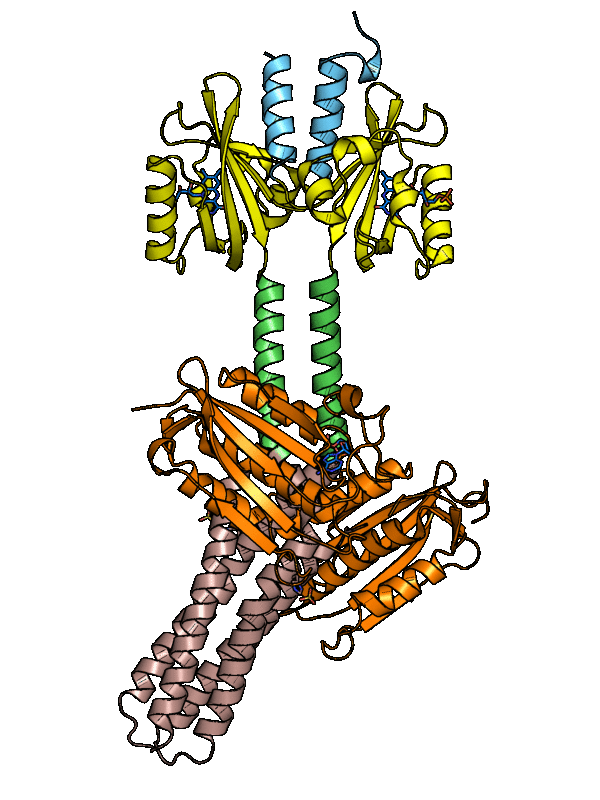 Figure - The three-dimensional structure of dark-adapted YF1 with LOV domains in yellow, DHp domains in
rose, CA domains in orange, the A'α helices in blue and the Jα helices in green.
Figure - The three-dimensional structure of dark-adapted YF1 with LOV domains in yellow, DHp domains in
rose, CA domains in orange, the A'α helices in blue and the Jα helices in green.
Informed by the high-resolution structure, we constructed variants of YF1 that alter its response to light. Of particular interest, several amino acid substitutions at the LOV:LOV dimer interface (e.g., D21V and H22P), led to inversion of the signal response and reprogrammed YF1 from a light-inhibited to a light-activated receptor.
- Möglich, A., Ayers, R.A., and Moffat, K. (2009). Design and signaling mechanism of light-regulated histidine kinases. J. Mol. Biol. 385, 1433–1444. [Pubmed]
- Diensthuber, R.P., Bommer, M., Gleichmann, T., and Möglich, A. (2013). Full-length structure of a sensor histidine kinase pinpoints coaxial coiled coils as signal transducers and modulators. Structure 21, 1127–1136. [Pubmed]
- Gleichmann, T., Diensthuber, R.P., and Möglich, A. (2013). Charting the Signal Trajectory in a Light-Oxygen-Voltage Photoreceptor by Random Mutagenesis and Covariance Analysis. J. Biol. Chem. 288, 29345–29355. [Pubmed]
Light-induced Conformational Transitions in YF1
A hallmark of most sensory photoreceptors is their completely reversible action, meaning that the light-induced signaling state is only transiently populated. As a consequence, structural analysis of the light-adapted states of photoreceptors by conventional means is challenging. To decipher at the molecular level the conformational transitions that underpin signal transduction in YF1, we resorted to two independent techniques. On the one hand, double electron-electron resonance (DEER) spectroscopy on spin-labeled receptor molecules provides precise local distance constraints. On the other hand, time-resolved X-ray solution scattering yields global structural information with medium resolution. Although conducted completely independently, both approaches consistently reveal light-induced splaying apart of the LOV photosensor dimer; this change likely feeds into the Jα coiled coil as left-handed supercoiling and enables regulation of effector activity.
- Berntsson, O., Diensthuber, R.P., Panman, M.R., Björling, A., Hughes, A.J., Henry, L., Niebling, S., Newby, G., Liebi, M., Menzel, A., Henning, R., Kosheleva, I., Möglich, A., Westenhoff, S. (2017). Time-resolved X-ray solution scattering reveals the structural photoactivation of a light-oxygen-voltage photoreceptor. Structure 25, 933-938. [Pubmed]
- Berntsson, O., Diensthuber, R.P., Panman, M.R., Björling, A., Gustavson, E., Hoernke, M., Hughes, A.J., Henry, L., Niebling, S., Takala, H., Ihalainnen, J.A., Newby, G., Kerruth, S., Heberle, J., Liebi, M., Menzel, A., Henning, R., Kosheleva, I., Möglich, A., Westenhoff, S. (2017). Sequential conformational transitions and α-helical supercoiling regulate a sensor histidine kinase. Nat. Comm. 8, 284. [Pubmed]
- Engelhard, C., Diensthuber, R.P., Möglich, A., and Bittl, R. (2017). Blue-light reception through quaternary transitions. Sci. Rep. 7, 1385. [Pubmed]
Regulating Transcription and DNA Cleavage by Light
 Suitably configured and embedded in cellular signaling networks, sensory photoreceptors mediate optogenetic control over DNA-associated processes, in particular
transcription and endonucleolytic cleavage. On the basis of the YF1 photoreceptor, we have devised efficient and portable systems for light-regulated gene expression
in prokaryotes. More recently, we also generated derivatives of the programmable, RNA-guided, sequence-specific DNA endonuclease Cas9 from Streptococcus pyogenes.
Learn less
Suitably configured and embedded in cellular signaling networks, sensory photoreceptors mediate optogenetic control over DNA-associated processes, in particular
transcription and endonucleolytic cleavage. On the basis of the YF1 photoreceptor, we have devised efficient and portable systems for light-regulated gene expression
in prokaryotes. More recently, we also generated derivatives of the programmable, RNA-guided, sequence-specific DNA endonuclease Cas9 from Streptococcus pyogenes.
Learn less
From Dusk Till Dawn
Together with its cognate response regulator BjFixJ, YF1 forms a light-regulated two-component system capable of regulating gene expression. To facilitate wide and easy deployment as an optogenetic tool, we constructed the one-plasmid systems pDusk and pDawn. Whereas in pDusk expression from the specific BjFixK2 promotor is repressed by around 15-fold under blue light, in pDawn it is strongly upregulated by around 450-fold under blue light.
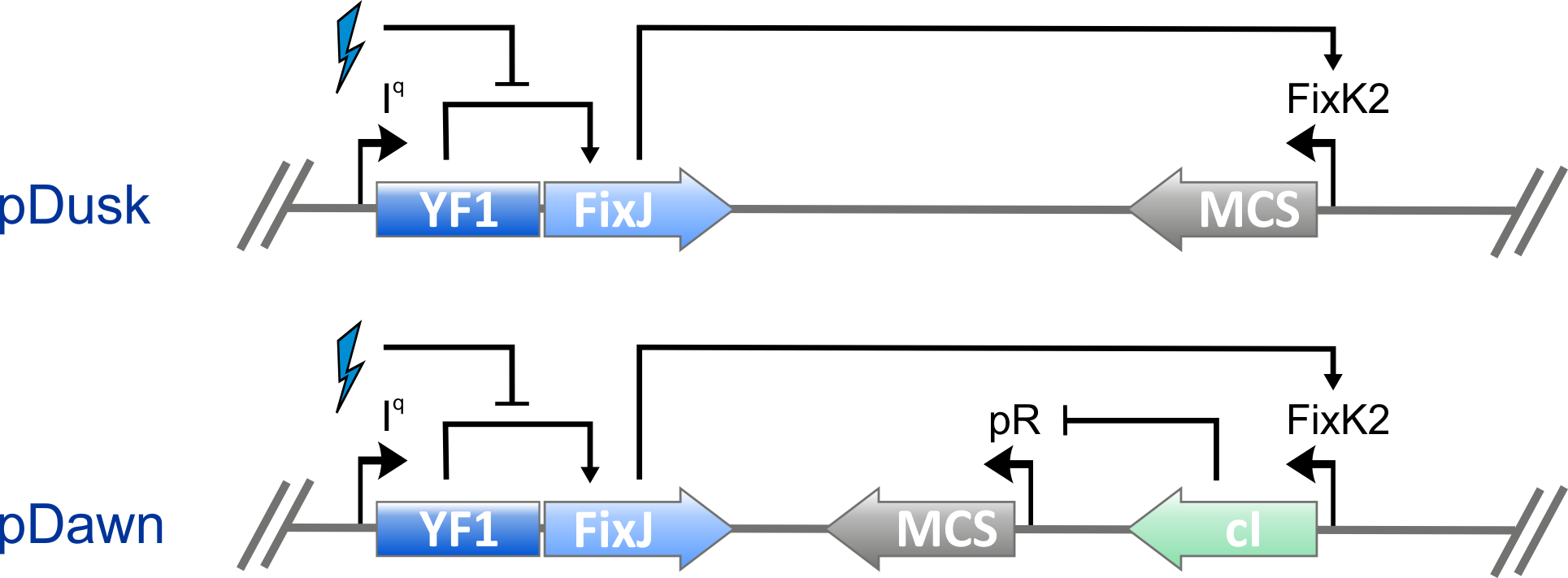
 Figure - In pDusk, YF1 drives blue-light-inhibited phosphorylation of BjFixJ; when phosphorylated,
BjFixJ in turn activates transcription from the BjFixK2 promotor. A multiple cloning site (MCS) allows insertion of desired target genes, e.g., the
red-fluorescent reporter DsRed from Discosoma sp. used here, under the control of this promotor. Signal inversion and amplification in pDawn is achieved
via insertion of the lamba repressor cI and its cognate promotor pR.
Figure - In pDusk, YF1 drives blue-light-inhibited phosphorylation of BjFixJ; when phosphorylated,
BjFixJ in turn activates transcription from the BjFixK2 promotor. A multiple cloning site (MCS) allows insertion of desired target genes, e.g., the
red-fluorescent reporter DsRed from Discosoma sp. used here, under the control of this promotor. Signal inversion and amplification in pDawn is achieved
via insertion of the lamba repressor cI and its cognate promotor pR.
- Möglich, A., Ayers, R.A., and Moffat, K. (2009). Design and signaling mechanism of light-regulated histidine kinases. J. Mol. Biol. 385, 1433–1444. [Pubmed]
- Ohlendorf, R., Vidavski, R.R., Eldar, A., Moffat, K., and Möglich, A. (2012). From dusk till dawn: one-plasmid systems for light-regulated gene expression. J. Mol. Biol. 416, 534–542. [Pubmed]
- Ohlendorf, R., Schumacher, C.H., Richter, F., and Möglich, A. (2016). Library-Aided Probing of Linker Determinants in Hybrid Photoreceptors. ACS Synth. Biol. 5, 1117-1126. [Pubmed]
- Stabel, R., Stüven, B., Ohlendorf, R., and Möglich, A. (2017). Primer-Aided Truncation for the Creation of Hybrid Proteins. Meth. Mol. Biol. 1596, 287-304. [Pubmed]
Cas9 Variants that Respond to Blue Light and Temperature
The programmable DNA endonuclease Cas9 has utterly revolutionized the modification and control of genomes. Specified by the sequence of a small accessory RNA molecule (denoted crRNA), Cas9 can be directed to arbitrary, unique sites in the genome where it cleaves both strands of the DNA double helix, thus eliciting downstream responses, principally homologous recombination and non-homologous end joining. A cleavage-incompetent variant of Cas9, denoted dCas9, serves as an RNA-guided, sequence-specific DNA-binding protein for transcriptional control and epigenetic modification of target loci.
We inserted into surface-exposed locations within Cas9 the homodimeric LOV domain from Rhodobacter sphaeroides which dissociates upon blue-light exposure. In this manner, Cas9 could be sequestered into an unproductive dimeric complex in the dark; blue light would promote dissociation and concurrent restoration of catalytic activity. From a library of > 105 variants, we selected one called paRC9 which showed a moderate light-induced increase in cellular dCas9 and Cas9 activities. From the same library, we also isolated tsRC9, a variant which displays robust activity at a permissive temperature of 29°C but hardly any activity at 37°C.
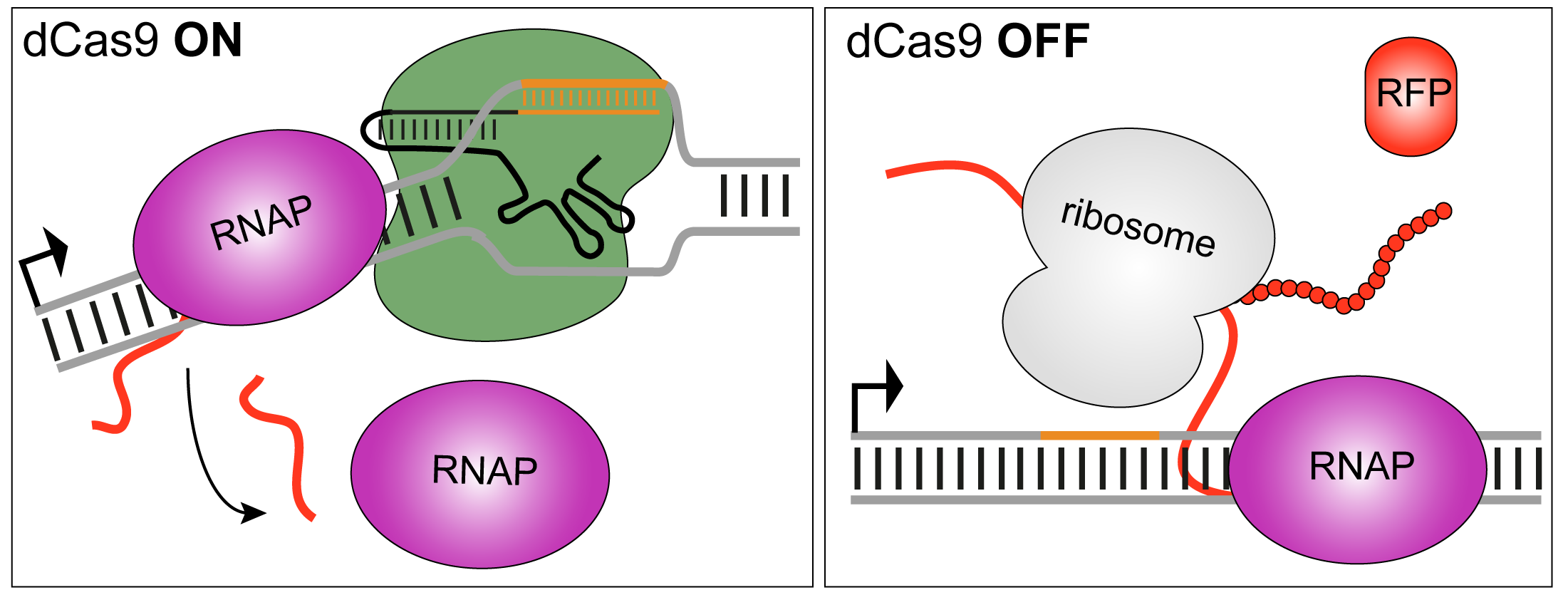
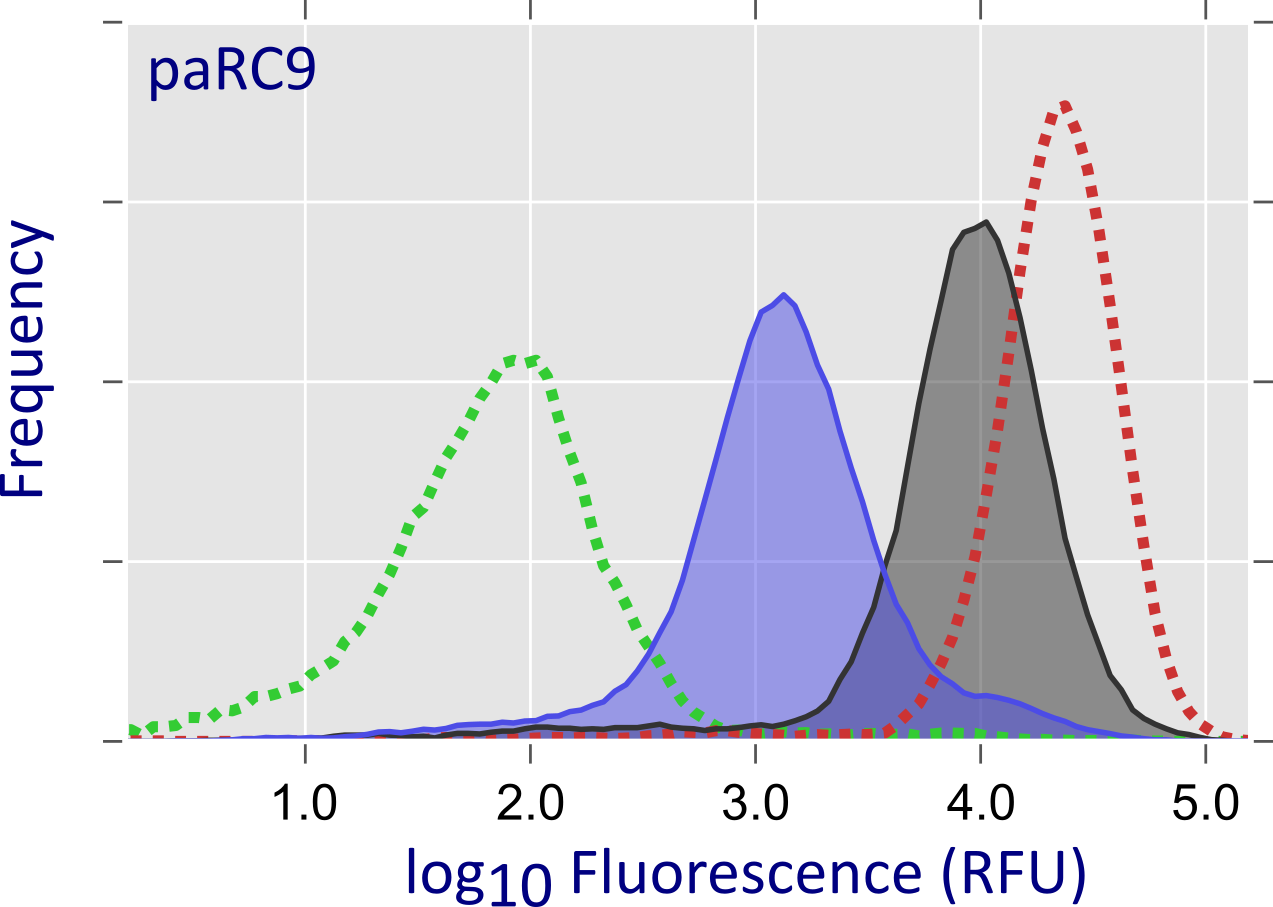
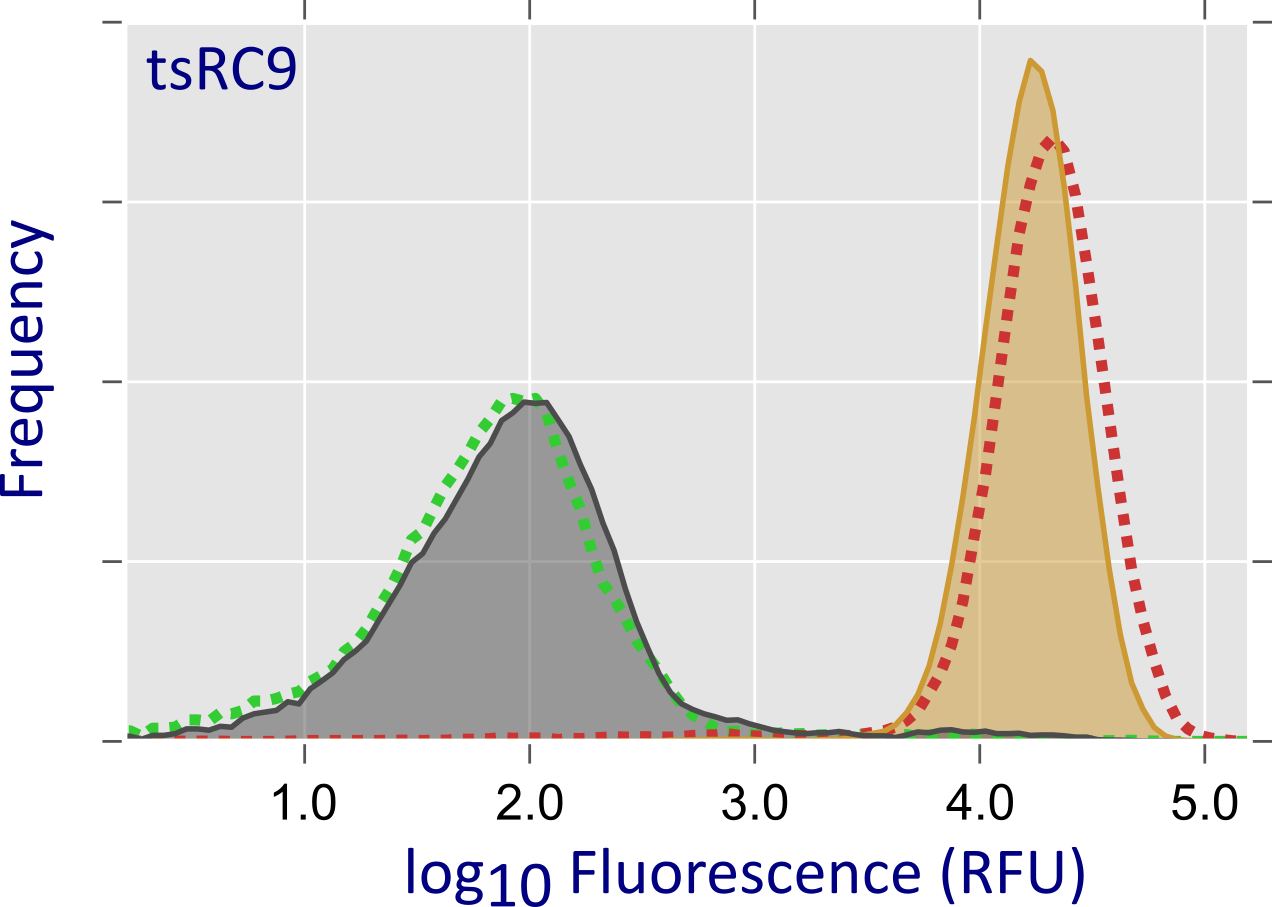 Figure - In a high-throughput screening assay, dCas9 is used as a transcriptional repressor for a red-fluorescent
reporter: upon dCas9 binding, reporter fluorescence is strongly reduced. Flow cytometry analysis indicates that the paRC9 variant represses transcription more efficiently under blue light
(shaded blue area) than in darkness (grey shaded area); dashed green and red curves are positive and negative controls, respectively. In case of tsRC9, repression at
29°C (grey shaded area) is almost as efficient as the positive control; by contrast, at 37°C (orange shaded area) transcriptional repression is essentially abolished.
Figure - In a high-throughput screening assay, dCas9 is used as a transcriptional repressor for a red-fluorescent
reporter: upon dCas9 binding, reporter fluorescence is strongly reduced. Flow cytometry analysis indicates that the paRC9 variant represses transcription more efficiently under blue light
(shaded blue area) than in darkness (grey shaded area); dashed green and red curves are positive and negative controls, respectively. In case of tsRC9, repression at
29°C (grey shaded area) is almost as efficient as the positive control; by contrast, at 37°C (orange shaded area) transcriptional repression is essentially abolished.
- Richter, F., Fonfara, I., Bouazza, B., Schumacher, C.H., Bratovič, M., Charpentier, E., and Möglich, A. (2016). Engineering of temperature- and light-switchable Cas9 variants. Nucl. Acids Res. gkw930. [Pubmed]
- Richter, F., Fonfara, I., Gelfert, R., Nack, J., Charpentier, E., and Möglich, A. (2017). Switchable Cas9. Curr. Opin. Biotech. 48, 119-126. [Pubmed]
Mechanism of Light-Oxygen-Voltage Receptors
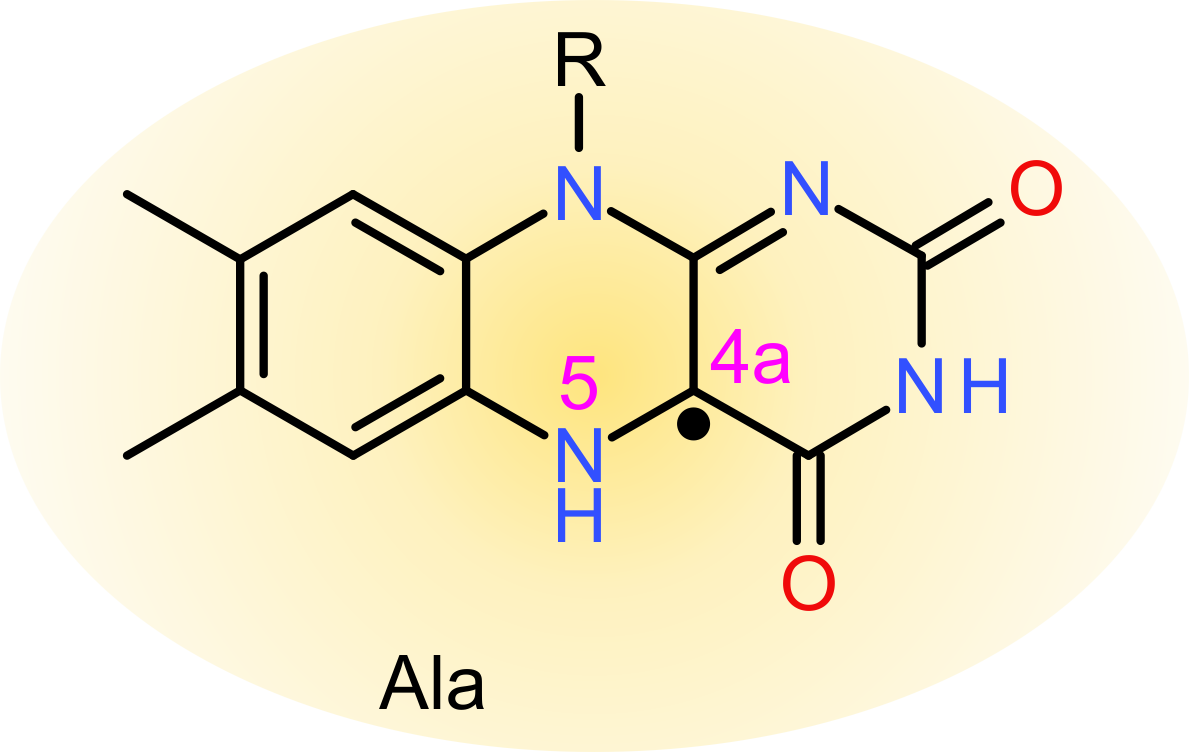 Notwithstanding numerous studies on naturally occurring LOV receptors and engineered representatives, certain principal aspects of photochemistry and signal
transduction still await full elucidation. To close these gaps in our understanding, we have characterized in detail the influence of the chromophore environment
even when their strictly conserved, active-site cysteine has been removed.
on photocycle and signal transduction in LOV receptors. In another study, we made the surprising finding that LOV receptors retain the ability to transduce signals
Learn less
Notwithstanding numerous studies on naturally occurring LOV receptors and engineered representatives, certain principal aspects of photochemistry and signal
transduction still await full elucidation. To close these gaps in our understanding, we have characterized in detail the influence of the chromophore environment
even when their strictly conserved, active-site cysteine has been removed.
on photocycle and signal transduction in LOV receptors. In another study, we made the surprising finding that LOV receptors retain the ability to transduce signals
Learn less
Influence of Chromophore Environment on Signal Transduction and Photocycle
In various light-oxygen-voltage photosensor modules, residues adjacent to the chromophore have been replaced in order to alter photocycle kinetics. Although such modifications are routinely and widely used to modulate response kinetics of light-gated actuators and effective light sensitivities at photostationary state, much less is known if and to what extent these modifications inadvertently impact correct signal transduction. Using YF1 as a model system, we characterized several widely used residue exchanges near the chromophore regarding their impact on downstream signal transduction.
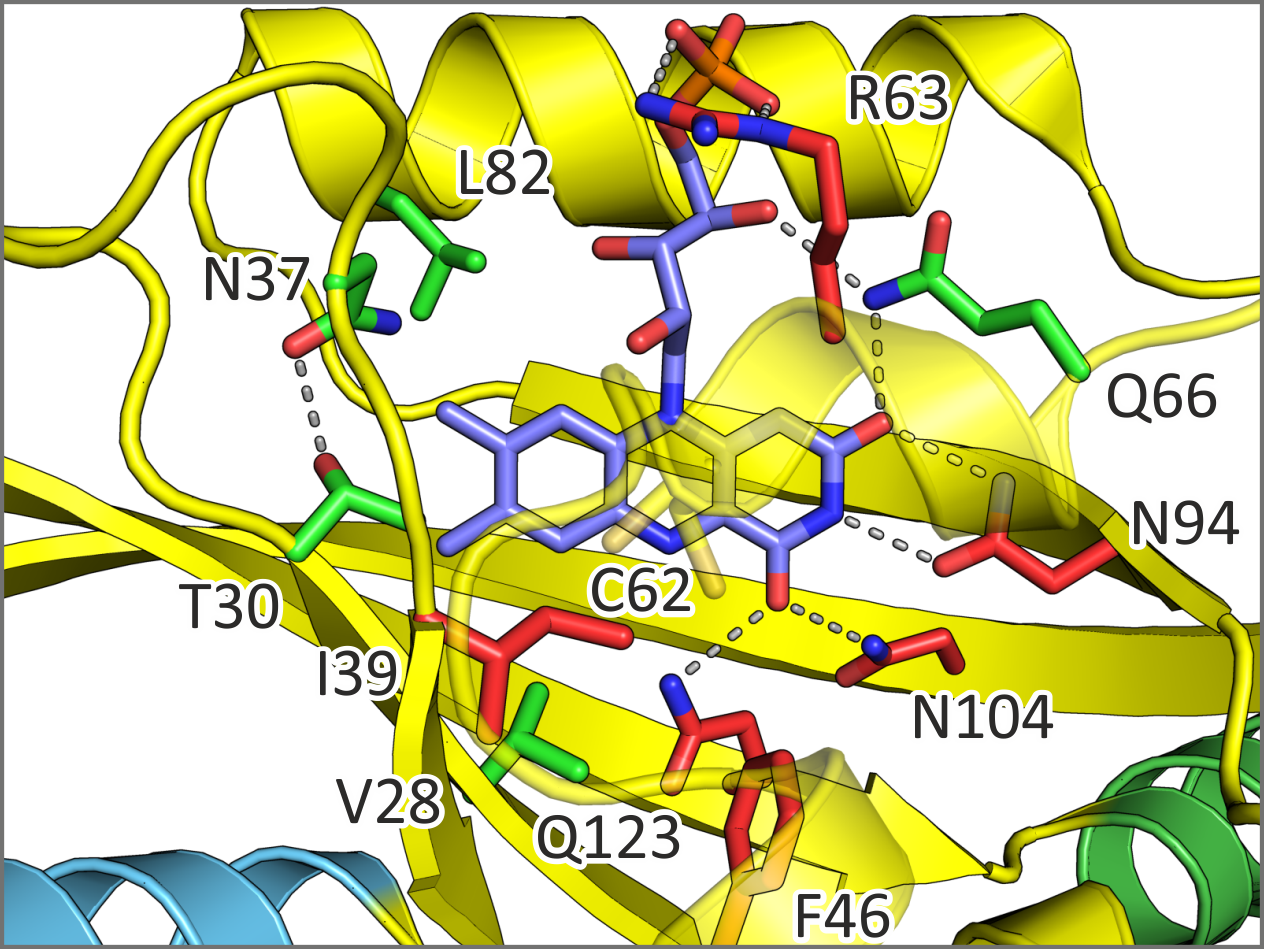 Figure - Certain residue exchanges near the flavin mononucleotide chromophore of YF1 are benign (green)
in that photocycle kinetics can be modulated while preserving intact signal transduction. Exchanges at other positions (red) impair correct signal transduction.
Figure - Certain residue exchanges near the flavin mononucleotide chromophore of YF1 are benign (green)
in that photocycle kinetics can be modulated while preserving intact signal transduction. Exchanges at other positions (red) impair correct signal transduction.
- Diensthuber, R.P., Engelhard, C., Lemke, N., Gleichmann, T., Ohlendorf, R., Bittl, R., and Möglich, A. (2014). Biophysical, mutational, and functional investigation of the chromophore-binding pocket of light-oxygen-voltage photoreceptors. ACS Synth. Biol. 3, 811–819. [Pubmed]
LOV, no Cysteines Attached
The active-site cysteine residue in LOV photoreceptors is strictly conserved, and in its absence, canonical photochemistry, in particular formation of a covalent thioether bond cannot occur. Unexpectedly, at least certain LOV receptors retain the ability to elicit downstream signal responses upon blue-light absorption, albeit at somewhat reduced light sensitivity. As a case in point, we showed that YF1 mediates light-dependent gene expression even after replacement of its active-site cysteine residue 62 by alanine.
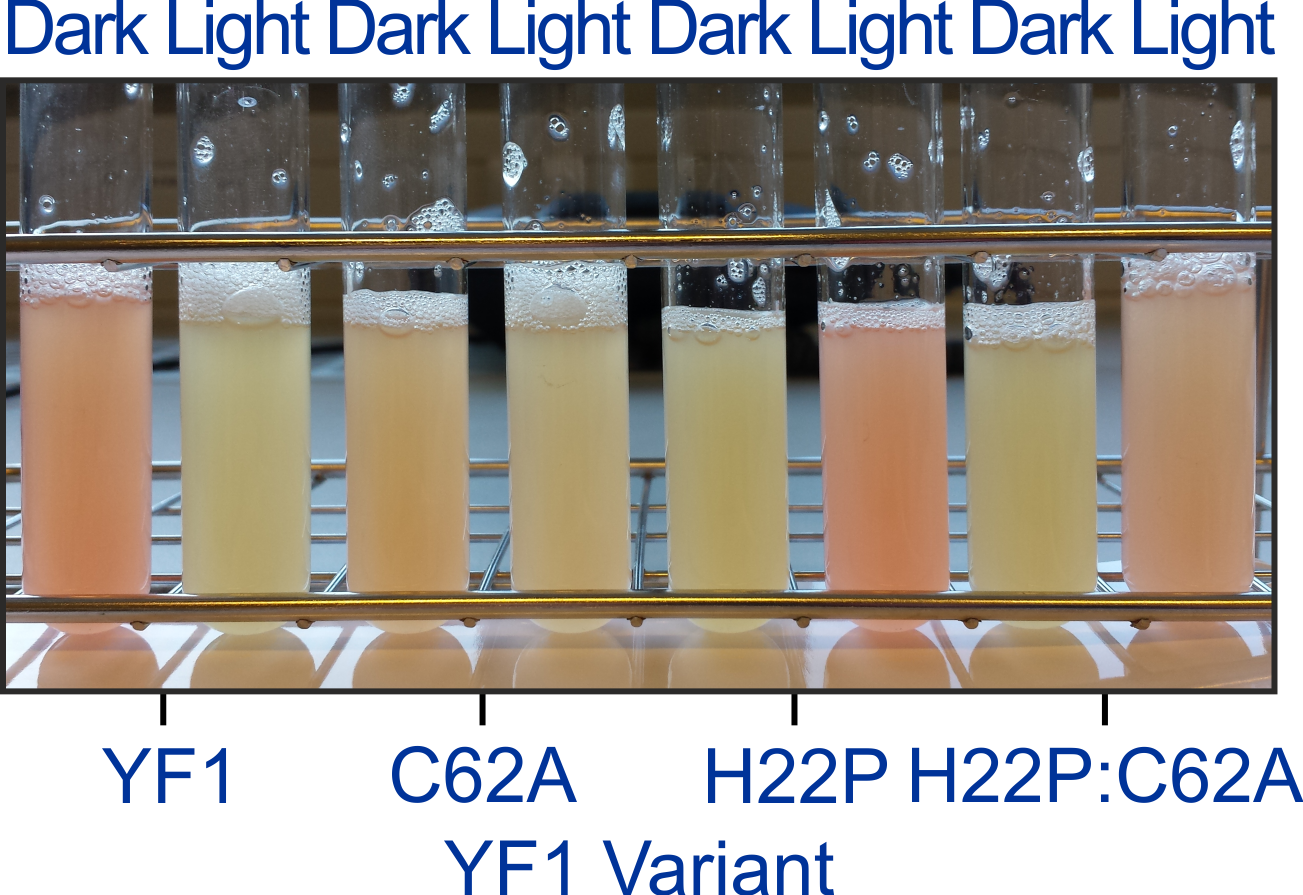 Figure - Despite replacement of the active-site cysteine C62 by alanine, YF1 is still capable of signal
transduction within the pDusk-DsRed reporter-system, in both the original YF1 and the signal-inverted H22P context.
Figure - Despite replacement of the active-site cysteine C62 by alanine, YF1 is still capable of signal
transduction within the pDusk-DsRed reporter-system, in both the original YF1 and the signal-inverted H22P context.
Detailed investigation of purified LOV receptors by absorption spectroscopy and electron paramagnetic resonance spectroscopy reveal that downstream signal transduction in cysteine-devoid LOV receptors is predicated on light-induced population of the partially reduced, neutral semiquinone (NSQ) radical state of the flavin nucleotide chromophore. In common with the thioadduct state of the parental, cysteine-containing receptors, the NSQ state also features a protonated N5 atom of the flavin isoalloxazine ring system. It is hence N5 protonation that is necessary and sufficient for downstream signaling in LOV receptors; by contrast, formation of the thioether bond per se is not strictly required.
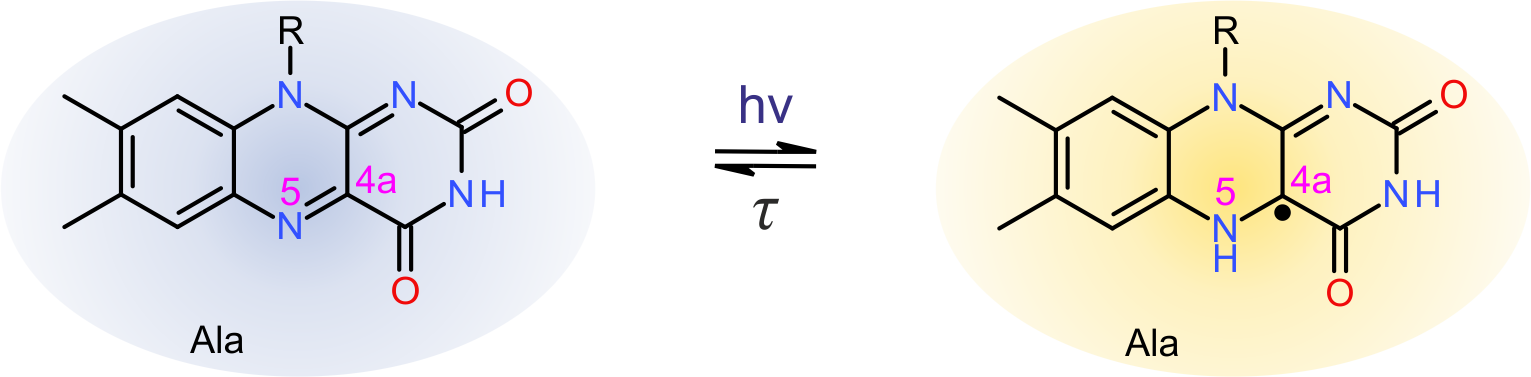 Figure - Alternate photocycle in cysteine-devoid light-oxygen-voltage (LOV) photoreceptors. Replacement of
the active-site cysteine by alanine abrogates thioadduct formation but abets blue-light-driven reduction of the flavin-nucleotide chromophore to the neutral
semiquinone (NSQ) state. Notably, the NSQ state is protonated at atom N5 and thereby elicits qualitatively the same downstream responses as cysteine-containing
LOV receptors upon adduct formation.
Figure - Alternate photocycle in cysteine-devoid light-oxygen-voltage (LOV) photoreceptors. Replacement of
the active-site cysteine by alanine abrogates thioadduct formation but abets blue-light-driven reduction of the flavin-nucleotide chromophore to the neutral
semiquinone (NSQ) state. Notably, the NSQ state is protonated at atom N5 and thereby elicits qualitatively the same downstream responses as cysteine-containing
LOV receptors upon adduct formation.
- Yee, E.F., Diensthuber, R.P., Vaidya, A.T., Borbat, P.P., Engelhard, C., Freed, J.H., Bittl, R., Möglich, A., and Crane, B.R. (2015). Signal transduction in light-oxygen-voltage receptors lacking the adduct-forming cysteine residue. Nat. Commun. 6, 10079. [Pubmed]
Optogenetic Control of Cyclic-Nucleotide Signaling
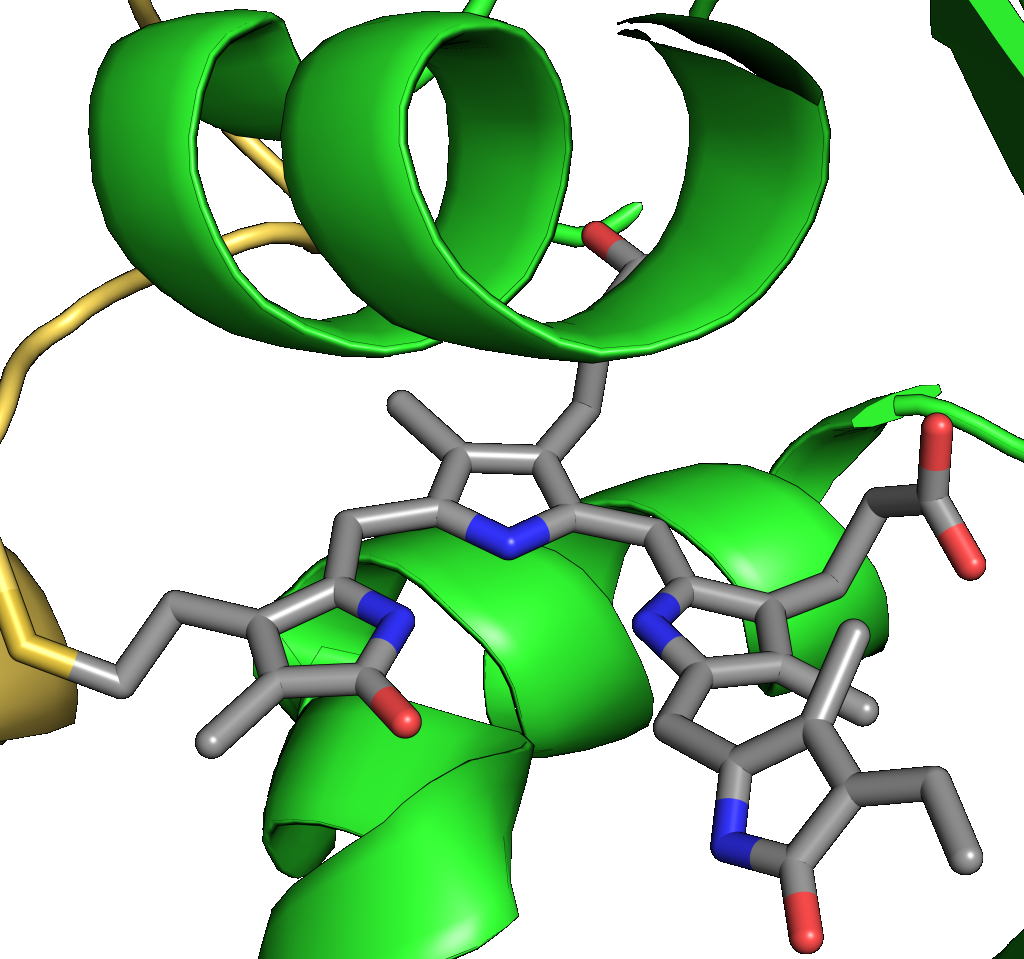 Several photoactivated nucleotide cyclases (denoted PAC) have been discovered in Nature that catalyze the formation of the universal second messengers 5',3'-cyclic
adenosine monophosphate (cAMP) and 5',3'-cyclic guanosine monophosphate (cGMP) in response to light. As cyclic nucleotide monophosphates (cNMP) are involved in the
regulation of numerous biological processes, PACs constitute veritable optogenetic tools that are finding frequent use. By contrast, no photoreceptor is known in
Nature that would directly catalyze the breakdown of cNMPs. To complement the optogenetic arsenal and to investigate signaling strategies of sensory photoreceptors,
we have engineered a phosphodiesterase that selectively degrades cAMP and cGMP in red-light-stimulated manner.
Learn less
Several photoactivated nucleotide cyclases (denoted PAC) have been discovered in Nature that catalyze the formation of the universal second messengers 5',3'-cyclic
adenosine monophosphate (cAMP) and 5',3'-cyclic guanosine monophosphate (cGMP) in response to light. As cyclic nucleotide monophosphates (cNMP) are involved in the
regulation of numerous biological processes, PACs constitute veritable optogenetic tools that are finding frequent use. By contrast, no photoreceptor is known in
Nature that would directly catalyze the breakdown of cNMPs. To complement the optogenetic arsenal and to investigate signaling strategies of sensory photoreceptors,
we have engineered a phosphodiesterase that selectively degrades cAMP and cGMP in red-light-stimulated manner.
Learn less
LAPD to the Rescue
Despite very distant evolutionary relationship, the three-dimensional structures of the PAS-GAF-PHY photosensor module of bacterial phytochromes (here, from Pseudomonas aeruginosa) and of the sensor module of certain mammalian phosphodiesterase (here, PDE2A from Homo sapiens) are remarkably similar. Both sensor entities adopt a parallel homodimer with an interface formed by α-helical bundles. Moreover, the laterally attached globular GAF and PHY domains are structurally highly similar. We figured that this structural resemblance might entail functional correspondence, that is, that one sensor module can functionally replace the other. To test this notion, we engineered LAPD (light-activated phosphodiesterase) by recombining the PAS-GAF-PHY domains of Deinococcus radiodurans bacteriophytochrome with the PDE effector domains of H. sapiens PDE2A.
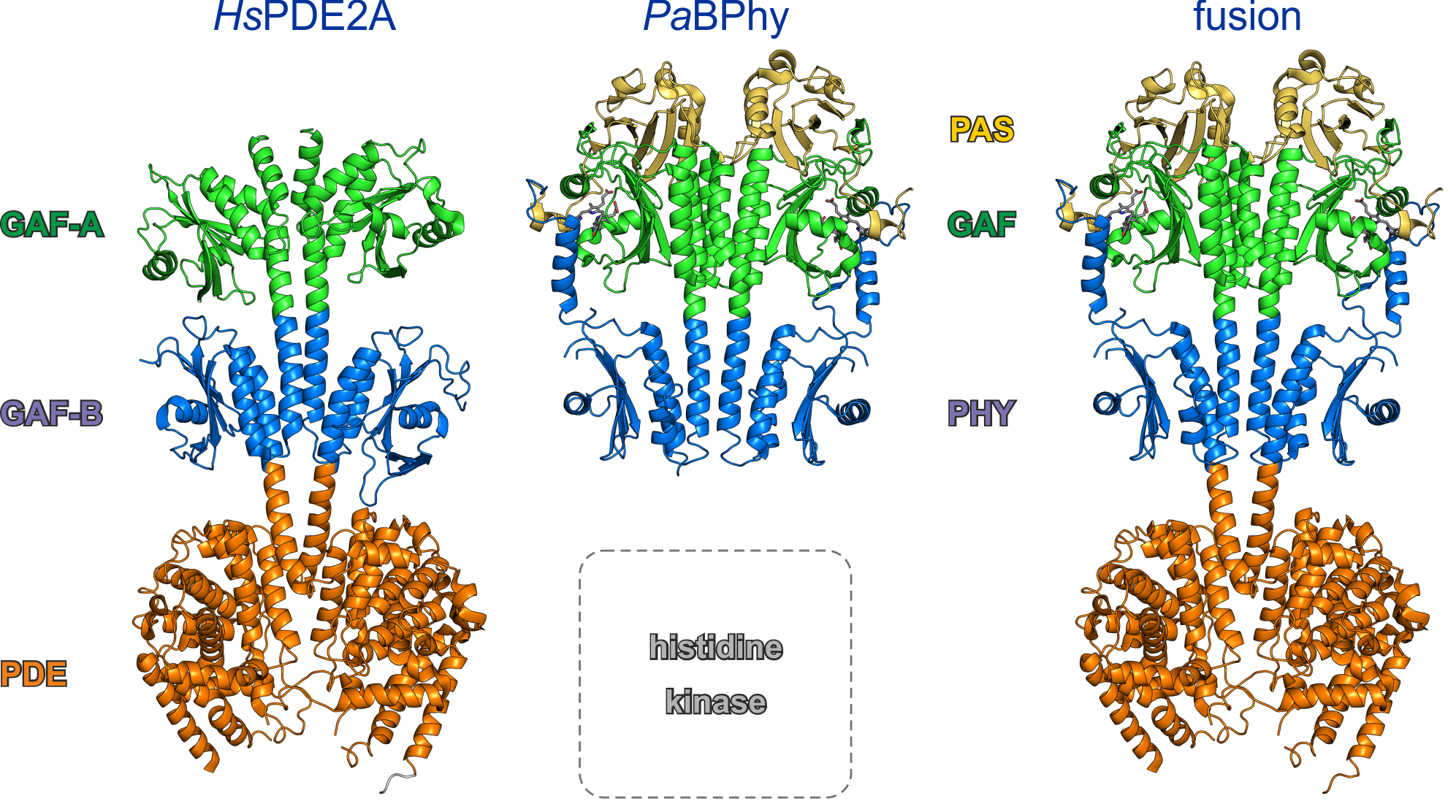 Figure - Bacterial phytochromes, e.g., from P. aeruginosa, and certain mammalian phosphodiesterases,
e.g., H. sapiens PDE2A, display remarkably similar three-dimensional structures of their respective sensor modules. We constructed LAPD by recombining the
PAS-GAF-PHY photosensor module from D. radiodurans bacteriophytochrome with the catalytic domain of HsPDE2A.
Figure - Bacterial phytochromes, e.g., from P. aeruginosa, and certain mammalian phosphodiesterases,
e.g., H. sapiens PDE2A, display remarkably similar three-dimensional structures of their respective sensor modules. We constructed LAPD by recombining the
PAS-GAF-PHY photosensor module from D. radiodurans bacteriophytochrome with the catalytic domain of HsPDE2A.
Measurements of cyclic nucleotide monophosphate (cNMP) hydrolysis showed that LAPD degrades cNMPs in red-light-stimulated manner. LAPD thus constitutes a hitherto unavailable implement that mediates an activity opposite that of PACs and that thus complements the optogenetic toolkit.
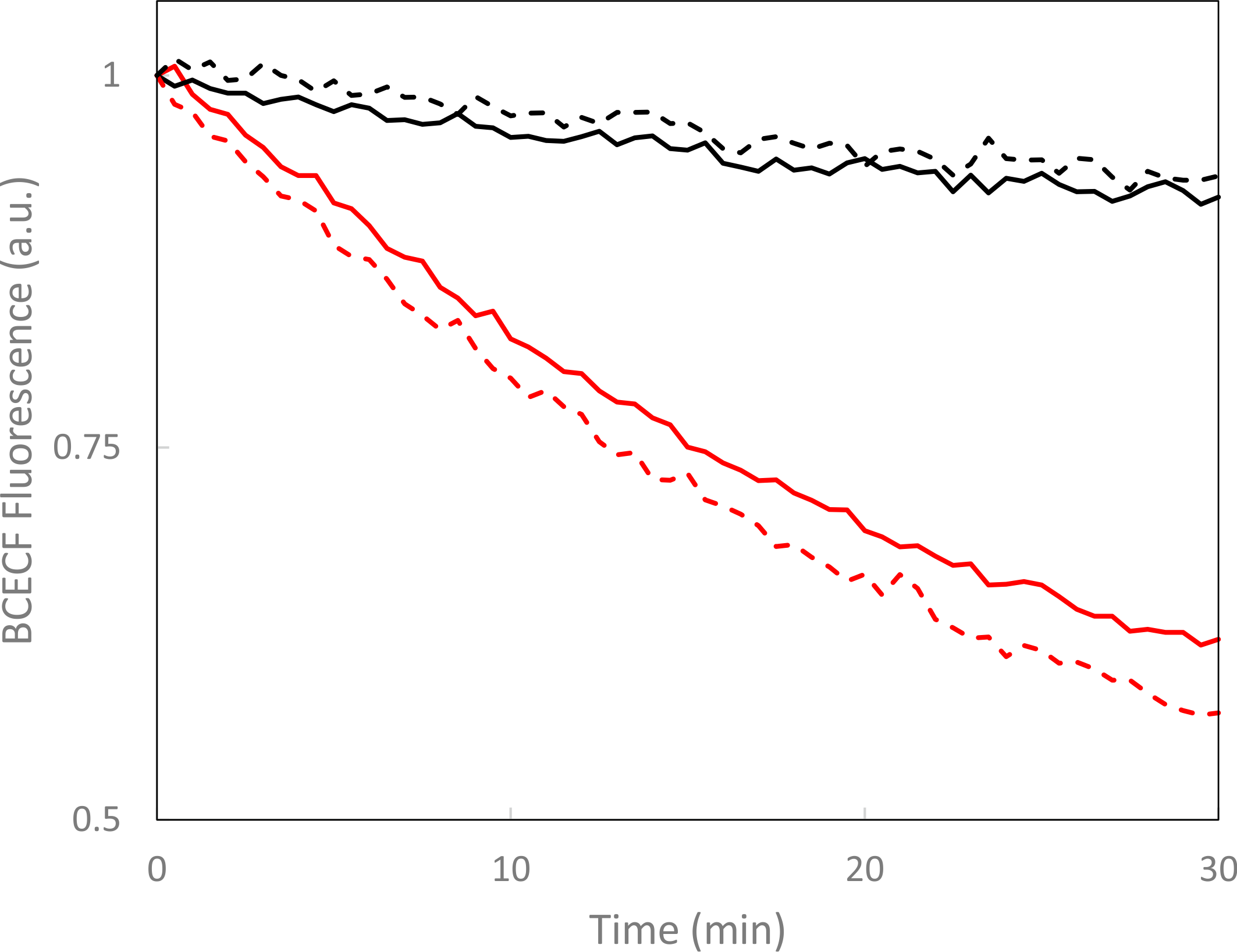 Figure - LAPD catalyzes the hydrolysis of cyclic nucleotide monophosphates in red-light-activated manner.
Turnover under dark (black) and red-light (red) conditions was measured by BCECF fluorescence for cyclic AMP (solid lines) and cyclic GMP (dashed lines).
Figure - LAPD catalyzes the hydrolysis of cyclic nucleotide monophosphates in red-light-activated manner.
Turnover under dark (black) and red-light (red) conditions was measured by BCECF fluorescence for cyclic AMP (solid lines) and cyclic GMP (dashed lines).
- Gasser, C., Taiber, S., Yeh, C.-M., Wittig, C.H., Hegemann, P., Ryu, S., Wunder, F., and Möglich, A. (2014). Engineering of a red-light-activated human cAMP/cGMP-specific phosphodiesterase. Proc. Natl. Acad. Sci. USA 111, 8803–8808. [Pubmed]
- Schumacher, C.H., Körschen, H.G., Nicol, C., Gasser, C., Seifert, R., Schwärzel, M., and Möglich, A. (2016). A Fluorometric Activity Assay for Light-Regulated Cyclic-Nucleotide-Monophosphate Actuators. Methods Mol. Biol. 1408, 93–105. [Pubmed]
- Stabel, R., and Möglich, A. (2017). Die Kontrolle zyklischer Nukleotide mittels Licht. BioSpektrum 23, 384-387. [doi]

